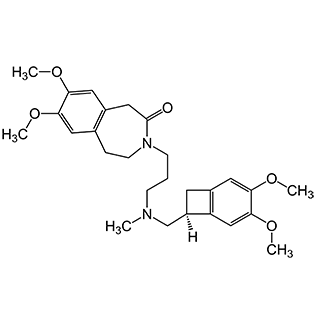
Ivabradine HCl
Ivabradine HCl (Hydrochloride) is a selective If (funny current) channel inhibitor active pharmaceutical ingredient widely used for the management of chronic heart failure and angina pectoris. By selectively reducing heart rate without affecting myocardial contractility, Ivabradine HCl decreases cardiac workload and oxygen demand, improving cardiovascular efficiency. Its unique mechanism of action makes it particularly valuable in patients who cannot tolerate or are inadequately controlled with conventional heart rate–lowering therapies. Pharmaceutical companies formulate Ivabradine HCl into oral tablets, providing precise and consistent dosing for long-term cardiovascular management.
Common Ivabradine HCl Uses and Treated Conditions
- Chronic Heart Failure – Reduces symptoms and improves exercise tolerance in patients with stable chronic heart failure.
- Angina Pectoris – Helps alleviate chest pain by lowering heart rate and myocardial oxygen consumption.
- Heart Rate Control – Provides selective heart rate reduction in patients with sinus rhythm.
- Adjunct Therapy – Used alongside standard heart failure or angina treatments for improved cardiovascular outcomes.
Why Choose Cambrex as Your Generic API Manufacturer?
As a leading generic API supplier, Cambrex manufactures Ivabradine HCl API in cGMP-compliant facilities, ensuring every batch meets stringent global quality and regulatory standards. Our technical expertise, robust quality systems, and proven regulatory track record empower clients to accelerate development timelines and achieve successful market approvals. We prioritize transparency, reliability, and scientific rigor at every stage of the supply chain making us your trusted generic API manufacturer.
For detailed chemical information on Ivabradine HCl, visit the official CAS website.
If you’d like more information or to request a sample, contact our Generic API team today.

Cambrex your API partner
Cambrex are a leading global supplier of generic APIs. From our sites in Italy, Sweden, and the USA, we work with generic drug companies well in advance of drug patent expiration, using high quality, non-patent-infringing processes to manufacture APIs.
Contact us
